Antibodies are part of the body’s adaptive immune response to defend itself against toxins and pathogens, such as bacteria and viruses. When the body identifies a foreign entity, it mounts a cascading response that culminates in the production of antibodies, which seek out and remove whatever initiated the response. Antibodies can employ a number of different mechanisms to inactivate the stimulus including, for example, enveloping small invaders, binding to and occupying specific sites of a foreign substance needed to interact with the host body, or binding a substance into an inactive conformation.
Antibodies were first used as therapeutics in 1986, and dozens have been approved since to treat a number of diseases, including autoimmune diseases and cancer. More recently antibodies have been developed to attack the SARS-CoV-2 coronavirus, which causes COVID-19.1,2
In the late 1980’s scientists envisioned a different use of antibodies in therapeutics when they recognized that the exquisite precision of antibodies might be used for targeted drug delivery.3,4 Today, such therapeutics are commonly referred to as antibody-drug conjugates (ADCs). ADCs offer a number of potential advantages over similar non-targeted therapeutics, including the possibility of increased efficacy, lower toxicity, and lower dosing requirements, but the complexity of ADCs creates significant development, regulatory, and intellectual-property hurdles that must be overcome.
The concept of targeted delivery of drugs is not new. As early as the 1900’s, Paul Ehrlich, who was awarded the 1908 Nobel Prize in Medicine in 1908, described “magic bullets” that would selectively deliver toxic substances to diseased tissue. Ehrlich recognized that diseased tissue was responsible for multiple diseases, most notably cancer.5 Removal of diseased tissue was necessary in most cases of cancer, but not always possible or completely accomplished with surgery. Systemic administration of toxic substances was capable of removing residual cancer, but the toxicity of those substances led to severe side effects. Ehrlich’s vision of targeted delivery might accomplish the goal of removing diseased tissue while minimizing side effects because the toxic substance would not act throughout the entire body.
However, the technology to realize this vision wouldn’t be available for almost a century. A revolution in monoclonal antibody research has opened the door to practical antibody-drug conjugates. But early research in the 1980’s led to less than desired results. These early efforts saw conjugation of known anti-cancer compounds methotrexate and doxorubicin to antibodies. Both failed in clinical trials for a number of reasons.
One reason was that these early ADCs employed antibodies that did not specifically target only cancer cells. In particular, the methotrexate and doxorubicin conjugates targeted sites that were also expressed by normal cells, albeit at a lower level. As a result, the conjugates were not as selective as hoped, leading to toxic side effects.
Another reason was that methotrexate and doxorubicin were not the most potent anticancer drugs, which allowed the drugs to migrate to normal tissues before reacting. Additionally, the moderate activity of these drugs meant more conjugate was needed. A more potent payload was needed.
The first approved ADC solved the payload problem by utilizing a much more potent cytotoxin. Mylotarg was the first approved ADC in 2000, using calicheamicin as the cytotoxic payload.6 Calicheamicin belonged to a class of recently discovered cytotoxic compounds known as enediynes, and was several thousand times more potent than doxorubicin.
A highly potent payload has several potential benefits. The first is decreased toxicity. A payload that is utilized immediately upon release has less time to migrate from the site of activation, and thus cause less systemic toxicity.
Another important feature of highly potent payloads is that fewer conjugates are required for activity. This is important for two reasons. One is simple economics: less payload is needed, so less ACD needs to be administered. The second reason is that highly potent payloads are much more amendable to ACD conjugation. Cytotoxic compound are generally highly non-polar and very poorly soluble. Attachment of multiple highly non-polar molecules to an antibody can significantly alter its conformation and binding affinity. A cytotoxic compound with only moderate activity could require such a large number of payload molecules on each antibody that the activity of the antibody is compromised significantly.
In addition, the low aqueous solubility of most cytotoxins means that fewer can be chemically attached to the antibody. Adjustments to increase the aqueous solubility of cytotoxins (addition of organic co-solvent, adjusting pH, etc.) can significantly alter the conformation of the antibody during conjugation. This could result in attachment of cytotoxin to normally inaccessible parts of the antibody, thereby trapping the cytotoxin and rendering it useless. In extreme cases, these conditions could cleave the antibody.
A further consideration is that the cytotoxin must contain a functional group on which to attach a linker that joins the cytotoxin to the antibody. If the linker is designed to leave a residual component on the payload, the cytotoxin must retain activity with this residual component attached.
The linker that attaches the cytotoxin to the antibody is designed to be cleaved at the target site. The ideal linker should be completely stable in blood and release the payload only at the targeted tissue (e.g., a tumor) or after antibody binding. Common antibody targets are cell surface receptors that cause the ADC to be internalized by the cell and conveyed to a lysosome in a process called receptor-mediated endocytosis. (Figure 1.)
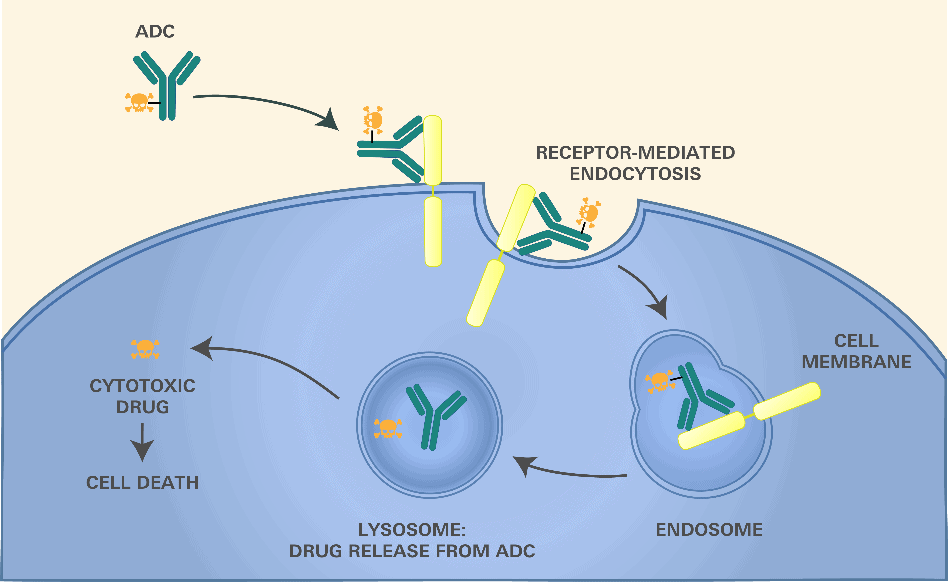
Figure 1.
The linker can take advantage of the differences in the environment of the lysosome—including the presence of certain enzymes, reduced pH, reductive environment, etc.—to selectively release the payload within the target cells. A number of linkers have been explored to take advantage of these differences.7
The important role of the linker is highlighted in the first ACD to be approved. Mylotarg was first approved in 2000 for treatment of acute myelogenous leukemia (AML).6 However, because of toxicity attributed to early release of drug due to an unstable linker, it was withdrawn in 2010. Mylotarg was re-approved in 2017 after evaluation of additional clinical data.
When designing linkers, choosing the sites of attachment on the antibody is crucial. The most common site of conjugation is the -amine of lysine residues of antibodies. But there are usually many lysines in an antibody, resulting in a high level of heterogeneity in the nature of ADCs. Cysteine conjugation is more attractive, as there are generally far fewer cysteines in an antibody. Lysine- and cysteine-based linkers can be cleaved in the lysosome by taking advantage of enzymes present in the lysosome or the reductive environment within lysosomes.
Cleavage at the lysine or cysteine of the antibody, however, usually leaves a residual component on the cytotoxic payload that may affect the payload molecule. As discussed above, the payload should be designed to accommodate this residual component.
Ideally the linker would be designed to cleave and leave no residual component on the cytotoxin.
Another consideration in linker design is that the linker and associated payload must not interfere with the binding affinity of the antibody. For example, a large non-polar cytotoxin in close proximity to the antibody may interfere with the binding of the antibody to its target. This can be ameliorated somewhat with a longer and/or hydrophilic linker.
In addition to the development considerations discussed above, there are multiple regulatory and intellectual property issues that affect ADCs.
The FDA regulates ADCs as biologics rather than as conventional chemically synthesized drugs.8 ADCs, therefore, are approved through a Biologics License Application (BLA) instead of the typical New Drug Application (NDA) for small-molecule drugs. While the BLAs are potentially more rigorous than NDAs (e.g., requiring an additional showing of product purity and potency), the BLA process offers at least two key benefits to ADC developers.
First, it is significantly more difficult, expensive, and time-consuming for third-party competitors to obtain FDA approval for a follow-on copy of a biologic (i.e., a biosimilar). A generic small-molecule drug applicant need only demonstrate “bioequivalence” to an FDA-approved reference drug, whereas a biosimlar applicant must show that its product is “highly similar to the reference product” with “no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product.”9 Thus, an ADCs status as a biologic offers significant market protection against follow-on competition. This protection may be somewhat undercut, however, where an ADC utilizes a well-established antibody, linker, or payload that may make it easier or less costly for a competitor to develop a biosimilar.
Second, new biologic products are entitled to 12 years of market exclusivity—independent of any patent protection—during which the FDA will not approve any biosimilar.10 In contrast, new chemical entities are only eligible for 5 years of market exclusivity.11 Importantly, the 12-year exclusivity only applies to the “first licensure” of a biologic.12 The FDA has not made clear whether an ADC that utilizes a previously FDA-approved antibody will be able to obtain “first licensure” status. Under the plain language of the Biologics Price Competition and Innovation Act of 2009, however, if the formation of the conjugate results in a “change in safety, purity, or potency” of the already licensed antibody, the ADC should be considered a first licensure eligible for exclusivity.13
Finally, because ADCs contain at least three separate functional elements—antibody, linker, and payload—they lend themselves to significant patent protections; each of these elements may be patentable both separately and in combination with each other. For the same reason, however, a third-party patent to any one of these elements may be sufficient to block the manufacture or sale of the full ADC in the United States.14 This issue is exacerbated by the maze of difficult-to-navigate intellectual property rights that have been established in the pharmaceutical field. To minimize these issues, ADC developers should perform a thorough freedom-to-operate analysis—using experienced IP counsel with significant knowledge in biotechnology, immunology, and organic chemistry—to ensure that no third-party patents or patent applications will hinder the marketing of a new ADC.
There are currently multiple approved ADCs, all for treatment of various cancers. As shown in Table 1, these conjugates target different antigens and utilize a variety of cytotoxins. Most of cytotoxins are small molecules, with the exception of moxetumomab pasudotox, which employs a 38 kDa fragment of Pseudomonas exotoxin A.
Also evident from Table 1 is that the number of ACDs approved has been growing significantly in recent years as the technology improves.
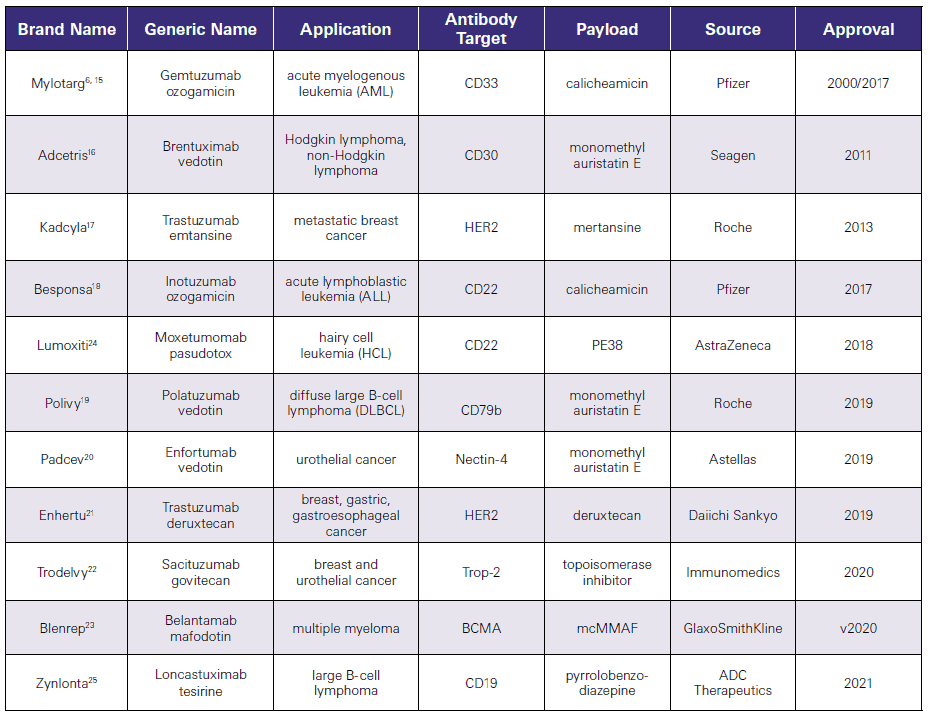
Table 1.
777 South Flagler Drive
Phillips Point East Tower, Suite 1000
West Palm Beach, FL 33401