June 25, 2025
On June 9th, 2025, the Federal Circuit issued a decision in Acadia Pharms. Inc. v. Aurobindo Pharma Ltd., affirming the district court’s grant of summary judgment of no invalidity for obviousness-type double patenting (OTDP) because the cited reference is not a proper ODTP reference.1 The Court applied the holding of a recent decision, Allergan USA, Inc. v. MSN Lab’ys Priv. Ltd., 111 F.4th 1358 (Fed. Cir. 2024), which held that “a first-filed, first-issued, later-expiring claim cannot be invalidated by a later-filed, later-issued, earlier-expiring reference claim having a common priority date.”2
MSN Laboratories PVT. Ltd. and MSN Pharmaceuticals, Inc. (collectively, “MSN”) filed an Abbreviated New Drug Application (“ANDA”) for a generic version of the Parkinson’s drug Nuplazid® (pimavanserin).3 Acadia Pharmaceuticals, Inc. (“Acadia”) sued MSN, among others, for patent infringement in the District Court of Delaware.4 By the time of the district court’s decision, the parties had agreed to limit the dispute to claim 26 of U.S. Patent No. 7,601,740 (“the ’740 patent”).5
MSN asserted that claim 26 of the ’740 patent, which is directed to a tartrate salt of pimavanserin, is invalid for OTDP in view of claim 5 of U.S. Patent No. 9,566,271 (“the ’271 patent”), which is directed to a method of treating hallucinations or delusions comprising administering a tartrate salt of pimavanserin.6 The parties stipulated that if the ’271 patent was a proper ODTP reference to the ’740 patent, then claim 5 of the ’271 patent anticipates claim 26 of the ’740 patent.7
The ’740 patent issued from Application No. 10/759,561 (“the ’561 application”) filed on January 15, 2004, and was granted on October 13, 2009.8 The 20 year term of the ’740 patent would expire on January 15, 2024, without any statutory extensions. However, the ’740 patent was also granted 980 days of patent term adjustment (PTA) under 35 U.S.C. § 154(b) for delays in the patent examination process, extending the patent term to September 21, 2026, and 1315 days of patent term extension (PTE) under 35 U.S.C. § 156 for the FDA review period of Nuplazid®, further extending the patent term to April 29, 2030.9
The ’271 patent issued on February 14, 2017, claiming priority to the ’561 application.10 The ’271 patent expired on January 15, 2024.11
A timeline of the salient dates of the ’740 and ’271 patents are provided below: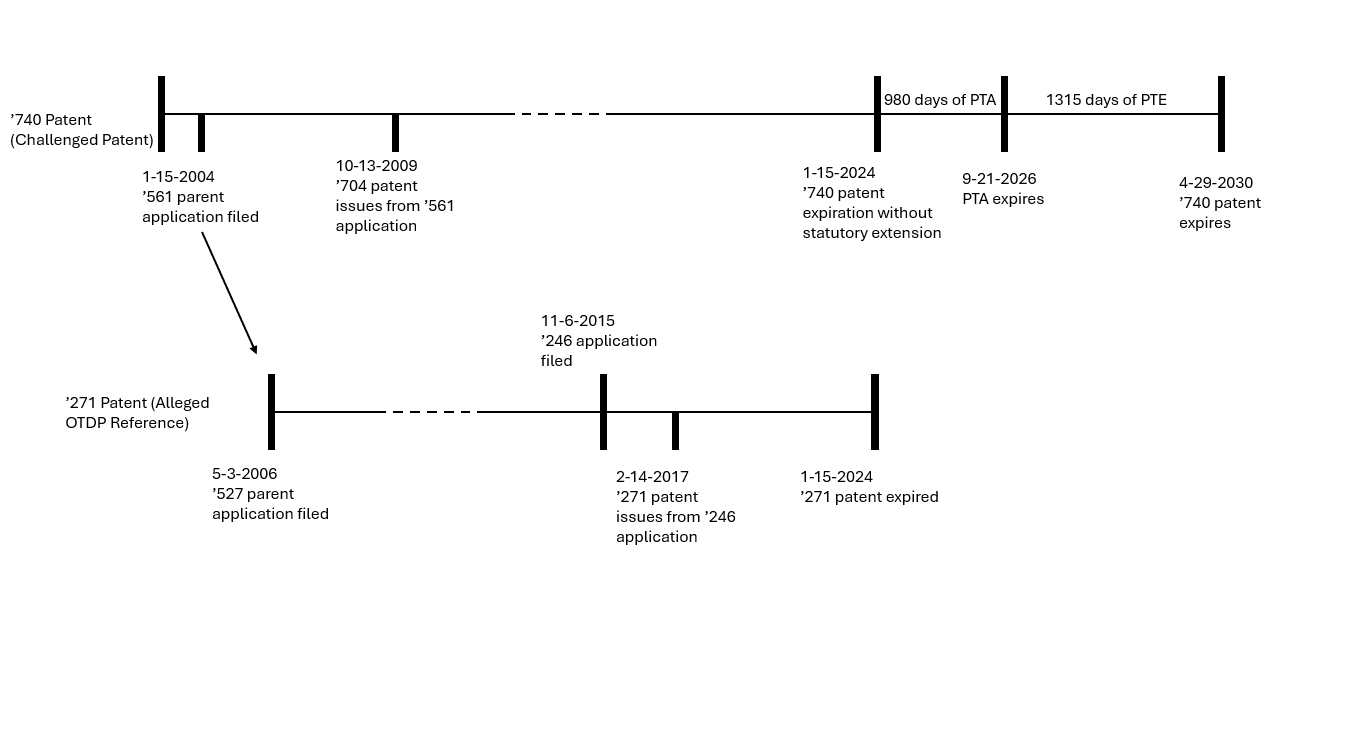
In a summary judgment motion, MSN challenged the validity of claim 26 of the ’740 patent under the judicially created doctrine of ODTP, which was established “to prevent an inventor from securing a second, later expiring patent for the same invention,”12 in view of claim 5 of the ’271 patent.
The district court explained that “[w]hether a patent qualifies as an OTDP reference is a threshold issue.”13 In analyzing the facts of this case, the district court held that “a patent must be earlier-filed to be available as an ODTP reference,” and therefore, “the ’271 patent does not qualify as a proper reference against the ’740 patent.”14 The district court distinguished this decision from the Federal Circuit’s decision in In re Cellect, 81 F.4th 1216 (Fed. Cir. 2023) because the patentee in that case opted not to challenge the availability of the reference patents being used in an OTDP challenge.15
MSN appealed the district court’s decision. After MSN filed for appeal, the Federal Circuit issued a precedential opinion in Allergan USA, Inc. v. MSN Lab’ys Priv. Ltd., holding that “a first-filed, first-issued, later-expiring claim cannot be invalidated by a later-filed, later-issued, earlier-expiring reference claim having a common priority date.”16
Both parties acknowledged that Allergen controlled, and therefore, MSN petitioned the Federal Circuit to hear the Acadia appeal en banc to overrule the precedent established in Allergan.17
Less than four months after MSN requested an en banc hearing and before holding oral arguments, a three-judge Federal Circuit panel issued a short opinion affirming the district court’s determination that the ’271 patent is not a proper ODTP reference to the ’740 patent, applying Allegan’s holding to this appeal.18
MSN requested and was granted an extension to file a petition for rehearing/en banc review to August 8, 2025.
The Federal Circuit’s decisions in Allergan and Acadia highlight the importance of challenging the propriety of a reference relied upon in an OTDP challenge. Both of these decisions provide patentees with some relief that, under certain circumstances, a later filed continuing application in the same patent family (i.e., having a common priority date) is not eligible as an OTDP reference. Nonetheless, patent applicants should remain cautious in continuation application filings, as the fact pattern of Allergan and Acadia is limited. The jurisprudence for OTDP remains in flux. It will be interesting to see whether the Federal Circuit grants MSN’s petition for rehearing/en banc review.
777 South Flagler Drive
Phillips Point East Tower, Suite 1000
West Palm Beach, FL 33401